6-Chloro-MDMA (Hydrochloride): In-Depth Analysis
Introduction to 6-Chloro-MDMA (Hydrochloride)
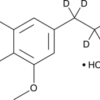
6-Chloro-MDMA (hydrochloride) is a chemical derivative of 3,4-methylenedioxy-N-methylamphetamine (MDMA), a well-known psychoactive compound. This modified version of MDMA contains a chlorine atom at the 6-position of its aromatic ring. This small alteration can lead to differences in its pharmacological effects, offering a unique tool for scientific research, particularly in the areas of pharmacology, neuroscience, and toxicology.
The compound is typically synthesized as a hydrochloride salt to improve its stability and handling properties. Understanding its structure, synthesis, applications, and how to acquire 6-chloro-MDMA (hydrochloride) is crucial for researchers and institutions engaged in pharmacological and forensic studies.
In this article, we will provide a detailed exploration of 6-chloro-MDMA (hydrochloride), including where to buy it in regions like Australia, the UK, Europe, and the USA for research purposes.
Chemical Structure and Properties of 6-Chloro-MDMA (Hydrochloride)
6-Chloro-MDMA (hydrochloride) shares a similar molecular structure with MDMA, but with one notable difference: the substitution of a chlorine atom at the 6-position of the aromatic ring. This modification may influence the way the molecule interacts with neurotransmitter receptors and other biological targets in the body.
Molecular Structure
The molecular formula of 6-chloro-MDMA (hydrochloride) is C10H13ClNO2·HCl. It consists of:
- A methylenedioxy group attached to a benzene ring.
- A nitrogen-containing ethylamine side chain.
- A chlorine atom substituted at the 6-position of the aromatic ring.
- The compound is typically found in its hydrochloride salt form, which is stable and crystalline.
This slight structural modification is thought to alter its pharmacodynamics compared to MDMA, which has been traditionally used for both recreational and therapeutic purposes.
Synthesis of 6-Chloro-MDMA (Hydrochloride)
The synthesis of 6-chloro-MDMA (hydrochloride) involves several steps of organic chemistry designed to introduce the chlorine atom into the MDMA structure. This process is usually carried out by professionals in chemical laboratories with expertise in organic synthesis.
1. Chlorination of MDMA
The chlorination process is typically conducted using chlorinating reagents such as chlorine gas or a chlorinating agent like sulfuryl chloride. Chlorination is carried out under controlled conditions to ensure the chlorine atom is placed specifically at the 6-position of the aromatic ring.
2. Formation of the Hydrochloride Salt
Once the chlorination step is completed, the compound undergoes purification to remove any excess reagents or byproducts. The final product is often crystallized in its hydrochloride salt form to increase its stability and ease of handling. The compound is then analyzed using techniques such as NMR spectroscopy or mass spectrometry to verify its structure and purity.
Pharmacological Properties of 6-Chloro-MDMA (Hydrochloride)
As a derivative of MDMA, 6-chloro-MDMA (hydrochloride) exhibits certain shared pharmacological properties but with potential modifications due to the chlorine atom. Research into its pharmacodynamics is ongoing, but it is expected to share similarities with MDMA in terms of serotonin, dopamine, and norepinephrine activity.
1. Serotonergic Activity
MDMA is well-known for its potent serotonin-releasing properties. It induces the release of serotonin in the brain, which is responsible for the feelings of euphoria, empathy, and emotional warmth that users often experience. The presence of chlorine in 6-chloro-MDMA may alter the way it interacts with serotonin receptors, potentially affecting its potency and duration of action.
2. Dopamine and Norepinephrine Effects
Like MDMA, 6-chloro-MDMA (hydrochloride) is likely to have dopaminergic and noradrenergic effects. It could increase the release of dopamine and norepinephrine, contributing to stimulant effects like increased energy and alertness. However, the presence of chlorine could influence how the compound interacts with dopamine and norepinephrine transporters, possibly altering its stimulant effects.
3. Toxicology and Side Effects
As with other MDMA analogs, the use of 6-chloro-MDMA (hydrochloride) may result in side effects, including hyperthermia, dehydration, serotonin syndrome, and elevated heart rate. Further research is necessary to fully understand the specific toxicological profile of 6-chloro-MDMA, but it is expected to share similar risks with MDMA use.
Applications of 6-Chloro-MDMA (Hydrochloride)
6-Chloro-MDMA (hydrochloride) serves as an important tool in scientific research, particularly in pharmacology, neuroscience, and toxicology. Below are some key applications of this compound:
1. MDMA Analogs Research
As an analog of MDMA, 6-chloro-MDMA is useful in the study of how structural modifications to the MDMA molecule affect its pharmacological profile. Researchers use this compound to explore how changes to the aromatic ring influence receptor binding, neurotransmitter release, and overall drug efficacy. These studies are essential for designing safer and more effective drugs targeting serotonin and other systems in the brain.
2. Drug Development and Design
The understanding of how chlorine substitution impacts MDMA-like compounds can aid in the development of new therapeutic agents. 6-Chloro-MDMA (hydrochloride) allows researchers to test the effects of such modifications on therapeutic outcomes, particularly in the context of mood disorders or other conditions where serotonin modulation is beneficial.
3. Forensic and Toxicological Studies
In forensic toxicology, 6-chloro-MDMA (hydrochloride) can be used as a reference standard to identify the compound in biological samples. This is particularly useful in drug testing and forensic investigations where the presence of MDMA derivatives needs to be identified. The compound’s role as a potential drug of abuse makes it important in drug screening and toxicology.
Where to Buy 6-Chloro-MDMA (Hydrochloride)
For researchers and institutions interested in purchasing 6-chloro-MDMA (hydrochloride), several reputable suppliers provide this compound. Below is a guide on where to buy it in key regions:
Buy 6-Chloro-MDMA (Hydrochloride) in Australia
In Australia, there are chemical supply companies that offer high-quality reference materials like 6-chloro-MDMA (hydrochloride) for research purposes. These companies provide the compound in small quantities for academic, forensic, or pharmacological studies. Shipping options and pricing may vary depending on the supplier.
Buy 6-Chloro-MDMA (Hydrochloride) in the UK
UK-based researchers can buy 6-chloro-MDMA (hydrochloride) from suppliers specializing in pharmaceutical-grade chemicals. Many of these suppliers offer international shipping and ensure that their products meet high standards of purity and quality. Trusted suppliers may include organizations like Sigma-Aldrich or Cerilliant.
Buy 6-Chloro-MDMA (Hydrochloride) in Europe
Across Europe, chemical distributors and scientific supply companies offer 6-chloro-MDMA (hydrochloride) for academic research. Whether in Germany, France, Spain, or other European countries, you can find reliable suppliers that provide this compound with assured purity. Many suppliers offer international shipping and customer support to ensure that researchers receive the correct product.
Buy 6-Chloro-MDMA (Hydrochloride) in the USA
In the USA, researchers can buy 6-chloro-MDMA (hydrochloride) from a variety of chemical supply companies. Major suppliers in the USA, such as Fisher Scientific, provide access to this compound in accordance with US research regulations. These suppliers offer secure shipping options, ensuring that the compound is delivered safely for scientific use.
Safety and Handling of 6-Chloro-MDMA (Hydrochloride)
Handling 6-chloro-MDMA (hydrochloride) requires adherence to strict safety protocols to prevent exposure to hazardous chemicals.
1. Personal Protective Equipment (PPE)
Researchers should wear personal protective equipment, including gloves, safety goggles, and lab coats, to minimize the risk of exposure to 6-chloro-MDMA (hydrochloride). Proper ventilation in the laboratory is also recommended.
2. Storage Conditions
6-Chloro-MDMA (hydrochloride) should be stored in a cool, dry place, away from direct sunlight, heat, and moisture. The compound should be kept in airtight containers to maintain its integrity and prevent degradation.
3. Disposal Procedures
Any waste or unused 6-chloro-MDMA (hydrochloride) should be disposed of following local regulations for chemical disposal. It is essential to use designated chemical waste disposal containers to prevent contamination and ensure safety.
Conclusion
6-Chloro-MDMA (hydrochloride) is a valuable compound in scientific research, particularly in the study of MDMA analogs, pharmacology, and toxicology. With its chemical modification, it provides insights into how changes to the MDMA structure impact its pharmacological profile, offering researchers the opportunity to design safer and more effective therapeutic agents.
If you are looking to buy 6-chloro-MDMA (hydrochloride) for research purposes, trusted suppliers in Australia, the UK, Europe, and the USA offer this compound with reliable shipping and high-quality standards. Be sure to follow all necessary safety precautions when handling and disposing of this substance to ensure safe research practices.
| 6-chloro-MDMA (hydrochloride) | 1Gram, 5Grams, 10Grams |
|---|
Be the first to review “6-Chloro-MDMA (Hydrochloride)” Cancel reply
Related products
MDMA Research Chemicals
MDMA Research Chemicals
MDMA Research Chemicals
MDMA Research Chemicals
MDMA Research Chemicals
MDMA Research Chemicals
MDMA Research Chemicals


Reviews
There are no reviews yet.